Empirical Formula for Molecular Formula :
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
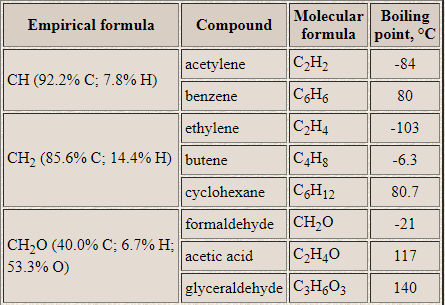
`color{blue} ul(mathtt ("EMPIRICAL FORMULA"))` : It represents the simplest whole number ratio of various atoms presents in a compound.
`color{blue} ul(mathtt ("MOLECULAR FORMULA"))` Molecular Formula : It is the exact number of different types of atoms present in molecule of a compound.
Method for determining the empirical and molecular formula can be learnt from the following examples :
`=>` Difference Between molecular mass and formula mass
1. Formula mass of a molecule is the sum of the atomic weights of atoms in its empirical formula.
2. Molecular mass of a molecule is its average mass that is calculated by adding together the atomic weights of atoms in the molecular formulae.
3. So, the main difference between the above two depends on whether we use empirical formula or molecular formula.
`●` The formula mass & molecular mass of `H_2O` are one & the same, where the formula & molecular mass of Glucose are different from other.
`color{blue} ul(mathtt ("EMPIRICAL FORMULA"))` : It represents the simplest whole number ratio of various atoms presents in a compound.
`color{blue} ul(mathtt ("MOLECULAR FORMULA"))` Molecular Formula : It is the exact number of different types of atoms present in molecule of a compound.
Method for determining the empirical and molecular formula can be learnt from the following examples :
`=>` Difference Between molecular mass and formula mass
1. Formula mass of a molecule is the sum of the atomic weights of atoms in its empirical formula.
2. Molecular mass of a molecule is its average mass that is calculated by adding together the atomic weights of atoms in the molecular formulae.
3. So, the main difference between the above two depends on whether we use empirical formula or molecular formula.
`●` The formula mass & molecular mass of `H_2O` are one & the same, where the formula & molecular mass of Glucose are different from other.

















